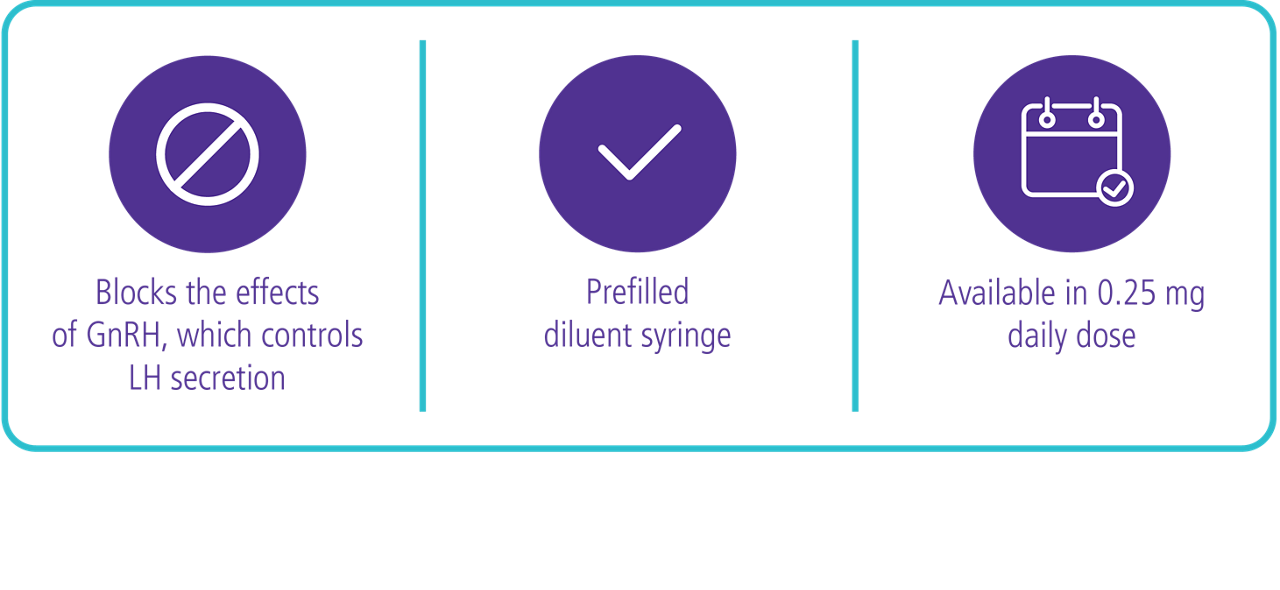
Cetrotide® (cetrorelix acetate for injection) is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian stimulation.
Clinical trial efficacy in OI and ART1
Study design
Seven hundred thirty-two (732) patients were treated with Cetrotide® (cetrorelix acetate for injection) in five (two Phase 2 dose-finding and three Phase 3) clinical trials. The clinical trial population consisted of Caucasians (95.5%) and Black, Asian, Arabian and others (4.5%). Women were between 19 and 40 years of age (mean: 32). The studies excluded subjects with polycystic ovary syndrome (PCOS), subjects with low or no ovarian reserve, and subjects with stage III-IV endometriosis.
Two dose regimens were investigated in these clinical trials, either a single dose per treatment cycle or multiple dosing. In the Phase 2 studies, a single dose of 3 mg* was established as the minimal effective dose for the inhibition of premature LH surges with a protection period of at least 4 days. When Cetrotide® is administered in a multidose regimen, 0.25 mg was established as the minimal effective dose. The extent and duration of LH-suppression is dose dependent.
In the Phase 3 program, efficacy of the single 3 mg dose regimen of Cetrotide®* and the multiple 0.25 mg dose regimen of Cetrotide® was established separately in 2 adequate and well-controlled clinical studies utilizing active comparators. A third non-comparative clinical study evaluated only the multiple 0.25 mg dose regimen of Cetrotide®. The ovarian stimulation treatment with recombinant FSH or human menopausal gonadotropin (hMG) was initiated on day 2 or 3 of a normal menstrual cycle. The dose of gonadotropins was administered according to the individual patient’s disposition and response.
In the single dose regimen study, Cetrotide® 3 mg* was administered on the day of controlled ovarian stimulation when adequate estradiol levels (400 pg/mL) were obtained, usually on day 7 (range day 5-12). If hCG was not given within 4 days of the 3 mg dose of Cetrotide®, then 0.25 mg of Cetrotide® was administered daily beginning 96 hours after the 3 mg injection until and including the day of hCG administration.
In the two multiple dose regimen studies, Cetrotide® 0.25 mg was started on day 5 or 6 of COS. Both gonadotropins and Cetrotide® were continued daily (multiple dose regimen) until the injection of human chorionic gonadotropin (hCG).
Oocyte pick-up (OPU) followed by in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) as well as embryo transfer (ET) were subsequently performed. The results for Cetrotide® are summarized below.
Results with Cetrotide®
| Parameter |
|
Cetrotide® 0.25 mg (md, active comparator study) |
Cetrotide® 0.25 mg (md, non-comparative study) |
| No. of subjects |
159 | 303 | |
| hCG administered [%] | 96.2 |
96.0 | |
| Oocyte pick-up [%] | 94.3 |
93.1 |
|
| LH-surge [%] (LH ≥ 10 U/L and Pa ≥ 1 ng/mL)b |
1.9 |
1.0 |
|
| Serum E2 [pg/ml] at day hCGc,d |
1064 (341-2531) |
1185 (311-3676) |
|
| Serum LH [U/L] at day hCGc,d |
1.5 (0.5-7.6) |
1.1 (0.5-3.5) |
|
| No. of follicles ≥ 11 mm at day hCGe |
10.8±5.2 | 10.4±4.5 | |
| No. of oocytes: IVFe_ ICSIe |
7.6±4.3 10.1±5.6 |
8.5±5.1 9.3±5.9 |
|
| Fertilization rate: IVFe |
0.62±0.26 | 0.60±0.26 |
aProgesterone
bFollowing initiation of Cetrotide® therapy
cMorning values
dMedian with 5th – 95th percentiles
eMean ± standard deviation
In addition to IVF and ICSI, one pregnancy was obtained after intrauterine insemination. In the five Phase 2 and Phase 3 clinical trials, 184 pregnancies have been reported out of a total of 732 patients (including 21 pregnancies following the replacement of frozen-thawed embryos).
The following common adverse reactions were reported at ≥1% in clinical trials.
| Adverse Events | Cetrotide® N=949 % (n) |
|---|---|
| Ovarian hyperstimulation syndromea | 3.5 (33) |
| Nausea | 1.3 (12) |
| Headache | 1.1 (10) |
aIntensity moderate or severe, or WHO Grade II or III, respectively.
Reference
1. Cetrotide® (cetrorelix acetate for injection) [Prescribing Information]. Rockland, MA: EMD Serono, Inc.; 2018.
IMPORTANT RISK INFORMATION & INDICATIONS AND USAGE
Important Risk Information for Cetrotide® (cetrorelix acetate for injection)
Cetrotide® is contraindicated in women who are pregnant, are nursing, or have severe renal impairment. Cetrotide® is also contraindicated in women who exhibit or have known hypersensitivity to cetrorelix acetate, mannitol, extrinsic peptide hormone, GnRH or any other GnRH analogs. Treatment with Cetrotide® is not advised in women with severe allergic conditions. Cases of hypersensitivity reactions, including anaphylactoid reactions have been reported. Other adverse events reported include ovarian hyperstimulation syndrome, nausea, headache, and local injection site reactions. Multiple births and congenital abnormalities have been reported.
Indication
Cetrotide® (cetrorelix acetate for injection) is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian stimulation. Cetrotide® should only be prescribed by physicians who are experienced with infertility problems and their management.
![]() For complete information, please see full Prescribing information here.
For complete information, please see full Prescribing information here.
IMPORTANT RISK INFORMATION & INDICATIONS AND USAGE
Important Risk Information for Cetrotide® (cetrorelix acetate for injection)
Cetrotide® is contraindicated in women who are pregnant, are nursing, or have severe renal impairment. Cetrotide® is also contraindicated in women who exhibit or have known hypersensitivity to cetrorelix acetate, mannitol, extrinsic peptide hormone, GnRH or any other GnRH analogs. Treatment with Cetrotide® is not advised in women with severe allergic conditions. Cases of hypersensitivity reactions, including anaphylactoid reactions have been reported. Other adverse events reported include ovarian hyperstimulation syndrome, nausea, headache, and local injection site reactions. Multiple births and congenital abnormalities have been reported.
Indication
Cetrotide® (cetrorelix acetate for injection) is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian stimulation. Cetrotide® should only be prescribed by physicians who are experienced with infertility problems and their management.
![]() For complete information, please see full Prescribing information here.
For complete information, please see full Prescribing information here.
